Hello everyone! I am Melaina Yender and this is my third week researching neuroscience and click chemistry at the Ye Lab at the Scripps Research Institute in San Diego, California. I have been conducting experiments with my mentors that allow us to trace drug targets in the brain through a process called clearing-assisted tissue click chemistry (CATCH). Last week I was granted the opportunity to conduct my own personal experiment with the CATCH process in order to gain a better understanding of how the process works. Alongside my experiment, I have been working with my mentors on a new Electrochem experiment for the past couple of weeks which will likely be continued through my internship.
This week I continued to complete my personal experiment by finishing the CLARITY and click protocols. At the beginning of the week, I divided the sliced tissue samples in half with the intention to only clear half of the collected tissues while leaving the other half uncleared. This was to compare the differences between cleared and normal tissue using confocal microscopy. For the samples I was going to clear, I created an A1P4 CLARITY gel solution for them to incubate overnight. The A1P4 gel is an acrylamide hydrogel and is responsible for keeping the cellular structure of the cells through polymerization. Following incubation, I began the polymerization process by degassing and removing all the oxygen molecules from the samples. This step is crucial because oxygen will quench the polymerization of the hydrogel by binding to the thermal initiator and bisacrylamide which would prevent the hydrogel from polymerizing. The degassing process happens with the samples in a vacuum chamber that removes all the air from the tubes. Then nitrogen is pumped into the chamber to essentially replace oxygen and prevent it from bonding to the polymers. These two steps and done a couple of times and then the samples sit in the chamber for 15 minutes to allow all oxygen to be removed. The samples can then polymerize at 37°C and left for about four hours. Then the samples can be transferred into 8% PBS-SDS for two days at 40°C. This is the step of the CLARITY process where the tissues become transparent and the lipids of the cells are removed. For the sake of time, I let the tissue samples sit in PBS-SDS for one day before moving on with the click labeling. This isn’t ideal as the slices weren’t fully cleared, but it wasn’t detrimental as this was primarily practice for me. After they were cleared, the tissues were washed a few times with PBST which is a detergent made with PBS and Triton X-100. I then stored the samples in a cold room in PBS with 0.02% NaN3. The CLARITY process is now finished at this point and the tissues are ready for click labeling.
For the click labeling process, I created an incubation buffer for the samples to incubate overnight. I selected one tissue from each tube of the cleared samples and from each of the not cleared samples for eight tissues in total. Each of the selected tissue had to be around a certain bregma or ‘depth’ in the brain. This is because we want to image the same part of each brain to compare the drug injected in parallel with the vehicle and to see how the drug binds to the brain compared to the vehicle. I aimed to select tissues around bregma 0.62 mm which is where the lateral ventricles begin to develop. The tissues were placed in a specially made rack with Eppendorf tubes taped underneath to slightly tilt the rack. This allows for the greatest amount of surface area of the tissue to be covered with the solution. This is done because there is only 195uL of solution for each tissue. The samples were incubated overnight on a shaker at room temperature. The following day I created the actual reaction buffer which is the same thing as the overnight buffer but includes sodium ascorbate which initiates the reaction. After that takes place, the reaction is then quenched with EDTA in PBS and then immediately washed with PBST multiple times again. After washing with the detergent, the tissues incubate in DAPI staining which is responsible for staining the nuclei in the tissue. This is done to see if the drug is actually binding to the cell rather than flooding the brain. This confirms that the drug is binding to the cell receptors and can then be pinpointed at what receptors it binds to.
The samples were then mounted onto a slide and imaged shortly after. There were a couple of problems noticed, and most of them were due to an issue with mounting the cover onto the slide. The cover was shifted and consequently shifted the tissues and caused problems with imaging. Vehicle #1 cleared and PF #1 cleared were not completely flat after the shifting of the cover which resulted in dark spots on the tissue. Vehicle #1 cleared and PF #2 cleared were shifted on the slide and were crooked which made imaging a little more difficult. Another problem was that the Vehicle #2 brain was not fully perfused and the blood vessels interfered with imaging quality. Finally, the Vehicle #1 brain was not oriented on the vibratome correctly which resulted in an imbalance of thickness through the slices. The brain was likely tilted in the agarose block and as the vibratome sliced the tissue, it did not cut it the same throughout. This is not ideal because the thicker the tissue, the more difficult it is to image and set the depth level for that individual slice. Regardless of the complications, the PF drug samples were notably bright compared to the Vehicle samples and the fluorescence of the drug molecules could be seen in the processed images. Overall, my experiment went well and it was seen as a success. I definitely have a thorough understanding of the CATCH process now and I am grateful to be given the opportunity to conduct my own experiment.
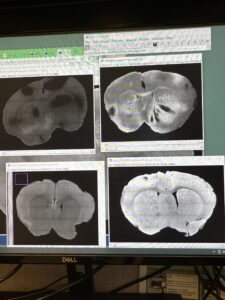
Processed results from Vehicle #1 samples (top and bottom left) and PF #1 samples (top and bottom right)
Aside from my experiment, my mentors and I attempted the Electrochem experiment for a third time and modified the procedure. The results from the previous week did not come back as intended for the in vivo samples and were not as great as the in vitro samples. It was expected that certain steps of the experiment were too aggressive and caused the probe to unbind from the receptors due to the weak bondage of the drug. We modified other steps as well and that would hopefully produce the results we wanted, which is an increase in the protein the drug bonded to in the electrically activated samples compared to the nonelectric samples. Unfortunately, the results came back the same for both the in vivo and in vitro samples. However, there was a difference between the electric and nonelectric samples for the in vitro which implied that the electrical activation caused stronger bondage of the drug to the proteins. This is huge as it suggests that the electrical activation is doing what we want by creating irreversible binding of the drug to the receptor. We will be attempting the experiment again this upcoming week and modifying it again.
This week was not as hectic as previous weeks but it was definitely busy. Outside of the lab I mostly spend time with the other Pinterns I’m staying with. In our downtime, we explored the area and have gone to downtown La Jolla and to Whispering Sands Beach quite often. We frequently go to many beaches to watch the sunset and have a fire in the backyard of our Airbnb. This week was a great success and I’m eagerly looking forward to next week!




Melania, we are so proud of you and everything you are accomplishing! It sounds like you are becoming very comfortable and finding your own path there! It makes us so happy that you have found what you love to do! The world is an open book to you! We love you so much!