Hey, guys! This is week two in the George Lab at the University of California, San Diego. Last week, I was warmly welcomed by all of the lab members and settled into the work environment, starting my new journey. Follow along for this week’s adventures!
Monday, July 19th, was a relatively calm day. I reported to Lisa Maturin, the Consortium Coordinator, and did more test tube labeling for designated post-dissection organs in the basement of Skaggs. This time, I labeled the tubes for a different cohort of rats – cohort 14. Each test tube is labeled with either an “M” or an “F” and four subsequent numbers. The letter indicates whether the organ in the tube belonged to a male or female rat. The first two numbers determine the cohort the rat was from. And the last two numbers indicate the specific rat in the cohort. For example, “F1429” shows that the organ in the tube was derived from a female rat, numbered “29”, in the 14th cohort. For the females, the codes are almost always “F1401” through “F1438”, and for the males, the combination is almost always “M1451” through “M1488”. Rarely, there’s an extra rat in the self-administration chambers, but when there is, the combinations adjust accordingly. After I finished labeling tubes, I traveled up to the fourth floor and had a long, insightful discussion with my mentor, Marsida. When I attended the 2021 July 12-14 INRC, I took lots of notes on the unpublished research, which I reviewed with Marsida here. I concentrated on three studies that I found the most interesting and presented all of my compiled data to her. She walked me through the deeper layers of each experiment: all of the fancy terms I didn’t know and concepts I didn’t quite grasp. We spoke about Von Frey filament tests which measure mechanical nociceptive threshold and plantar tests which measure thermal nociceptive threshold (in seconds) depending on paw withdrawal latency. We also discussed cFos expression, alcohol use disorder (AUD), binge-eating disorder (BED), and how COVID-19 has imposed a psycho-social impact on the population. (Note: Because all of these studies are unpublished, it’s strictly prohibited that I share any data about their results.)
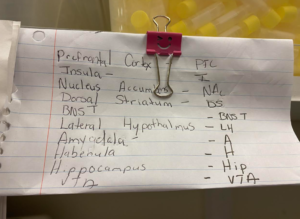
On Tuesday, I did even more test tube labeling, but this time, it was for regions of the brain. As Lisa explained, after dissecting specific rat brain tissue (aka “punching”), those various “punches” need to be distributed into tubes. I worked on the tubes designated for rats exposed to oxycodone. Because the brain is such a popular region of association to addiction, so many corporations want rat brain tissue. A complete set of brain punches requires 11 tubes, each for various abbreviated regions. I sorted sets of tubes for both male (blue) and female (clear) rats. Additionally, the George Lab is in the process of ordering more rats for a new cohort and new wave of experiments/trials, so after I finished labeling and differentiating all the tubes, I traveled upstairs to the Wet Lab to help make catheters for the new rats’ intravenous (IV) catheterization. In simple terms, IV catheterization is just a catheter inserted into a small peripheral vein for purposes such as administration of medications. In this case, the catheter will be inserted into the rats’ jugular veins and administer drugs like oxycodone and cocaine in self-administration studies. Because pre-assembled jugular vein catheters get to be pricey, the lab buys the parts of the catheter in bulk and makes them by hand, including the mold which is made out of a liquid monomer and a powder polymer (the stuff for acrylic nails). Then, once made, lab members later conduct intravenous jugular catheterization, and the catheter is inserted.
Wednesday was quite exciting! I finally got to see the rats, but with a twist – rat brain dissections. All of these rats had finished an entire round of experiments and it was time for them to travel to rodent nirvana. After euthanizing the rats via CO2 gas, the rat is then weighed, and their head is removed from the rest of their body. To then receive access to the brain, the skull needs to be carefully chipped away. After the bone has been mainly removed and the brain is exposed, the dura mater then needs to be peeled off. Its function is to protect the brain and spinal cord. Below the dura is the super thin and transparent arachnoid mater, which also needs to be peeled. After those 2 membranes are taken off and discarded, the rat’s brain is delicately extracted from the skull, and placed in a beaker of -20C to -30C 2-Methylbutane. The 2-Methylbutane is used with dry ice to freeze tissues for cryosectioning, which was taking place Thursday. After soaking in the 2-Methylbutane for a few minutes, the brain is then gingerly placed in a vial in a styrofoam box with dry ice, and that box is then stored in a freezer at -80C. After dissections, I accompanied senior research assistant Molly Brennan to one of the self-administration rooms in BSB. She showed me how to change the bedding of the rat cages and collect the rats’ urine for sampling and testing. This consumed the rest of my day as there are four rooms with many cages in each room!
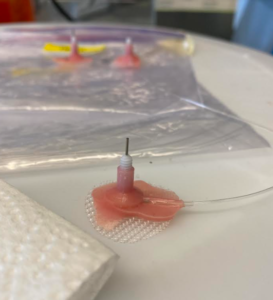
On Thursday, I went straight to BSB and worked with Brent Boomhower, a research assistant technician, who showed me and another volunteer, Nina Suh-Toma, how to fill syringes with oxycodone. As shown in the image on the left, a 30mL syringe is placed on the top of the cabinet with a tube running down into the self-administration chamber connected to a long wire. When a rat is in the cage during experiments, it is presented with a two-bottle paradigm. In a chamber, there are 2 levers; if a rat presses down on one lever, a certain drug is injected into its blood via IV catheterization from the 30mL syringe, and if a rat presses down on the other lever, saline is injected. Because many rats were pressing the drug lever several times due to their addicted state, the oxycodone syringes were quite low. Based on the rats’ weight (grams), Nina and I had to fill syringes with varying concentrations of oxy. For example, rat F1301 weighed 208g, so it fell into the mean group 220. Comparatively, rat M1353 weighed 353g, so it fell into the mean group of 340. It’s crucial to make sure that these rats received the correct concentration of oxycodone; if not, some rats would become intoxicated more easily than others and vice versa, unbalancing the ratios of the experiments. After Nina and I filled all of the syringes, we traveled to the Wet Lab in Skaggs and Angelica Martinez showed us what “mounting” brain slices looked like. Though a relatively simple task, you have to be quite delicate. For context, a single strand of hair is around 100μm. A rat brain slice is approximately 40μm, so they tear quite easily. To move brain slices around, we have to use a soft-bristled paintbrush to ensure that the slices don’t split. Essentially, mounting is where you pick out certain regions of the brain to “mount” onto a microscope slide for later examination by scientists and researchers.
When I wasn’t working, I was spending time with my dad and my new host family that I moved in with last weekend! My dad and I hiked along the Father Junipero Serra Trail and the Climber Loop Trail and visited Convoy Street, which had oriental food TO DIE FOR. A Pintern I’m rooming with and I have gone shopping at the mall, and with our host family, we’ve been watching the Olympics!




There are no comments published yet.